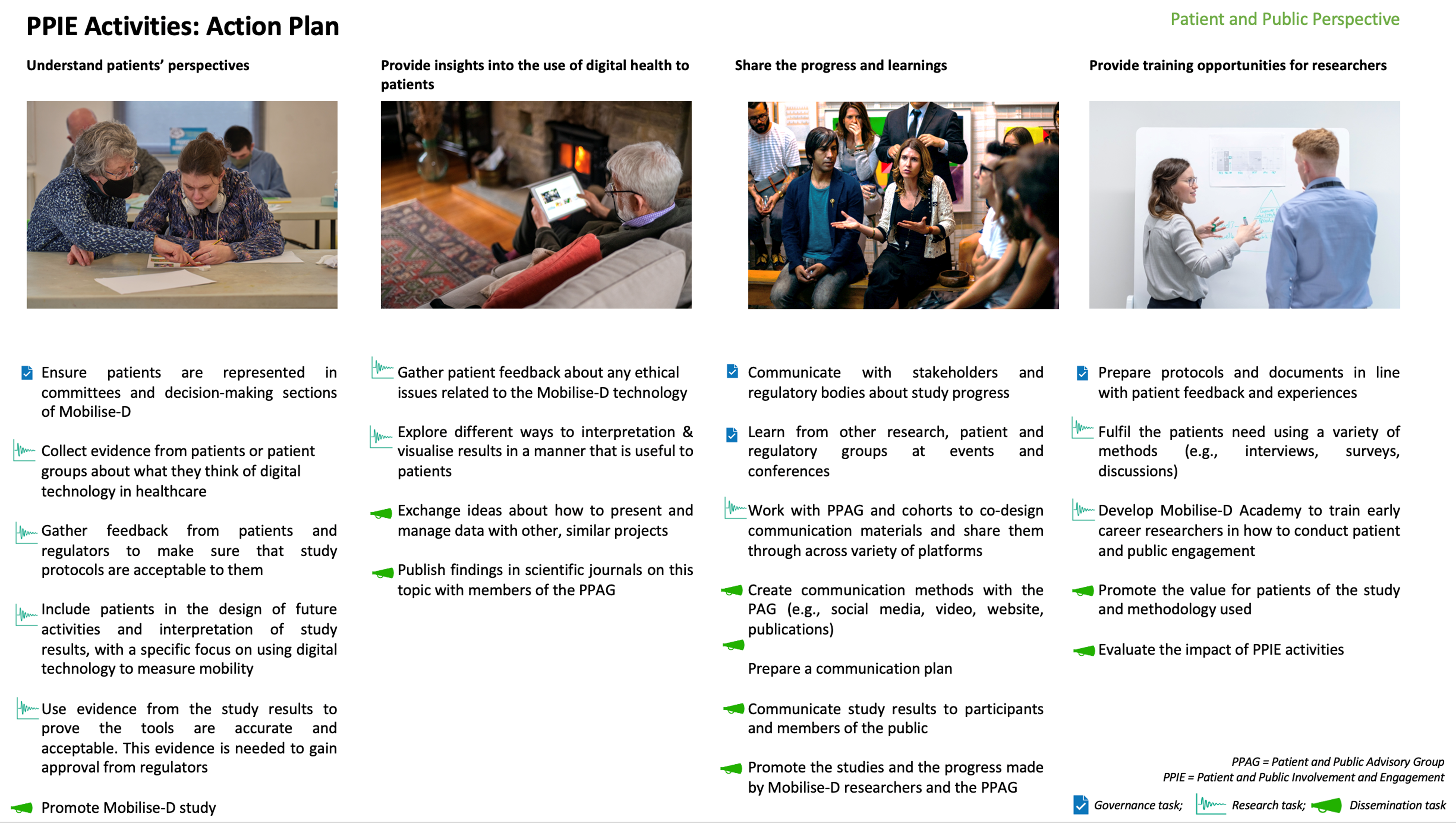
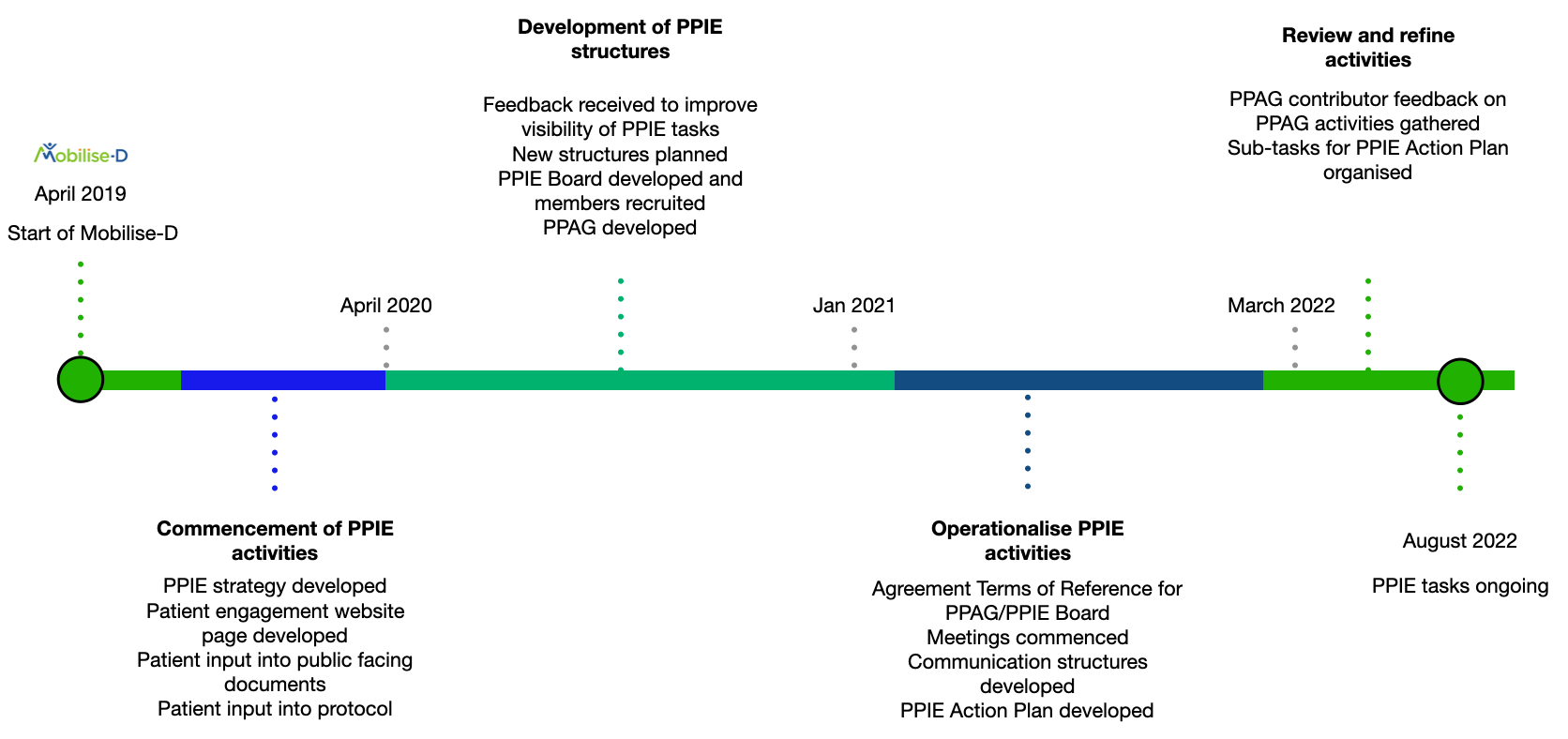
Within the roadmap for Mobilise-D, Patient and Public Involvement and Engagement (PPIE) was highlighted as a critical aspect of the work of the consortium. PPIE activities bring patient and public representatives into the research process to help researchers better understand lived experiences, to highlight areas of importance, and to support them in sharing their results in a way that ensures more people can both access and understand them.
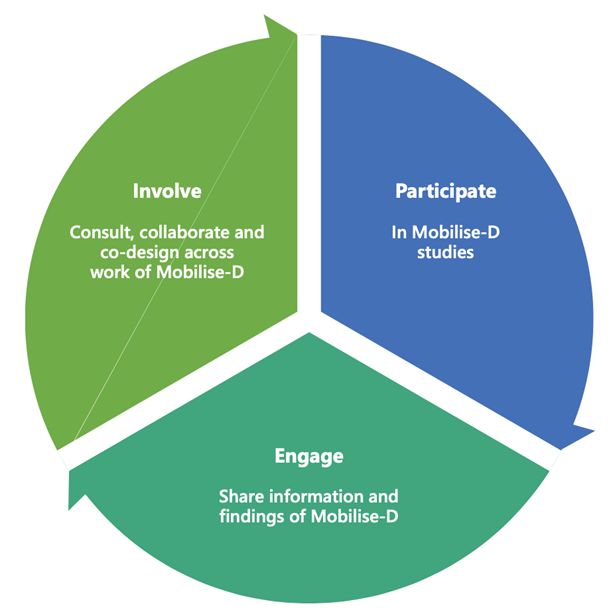
Patient and public representatives are often involved in research projects in three areas: involved in the design of the research, participating in research studies and engaging with information about research findings and progress (see figure below – Principles of PPIE).